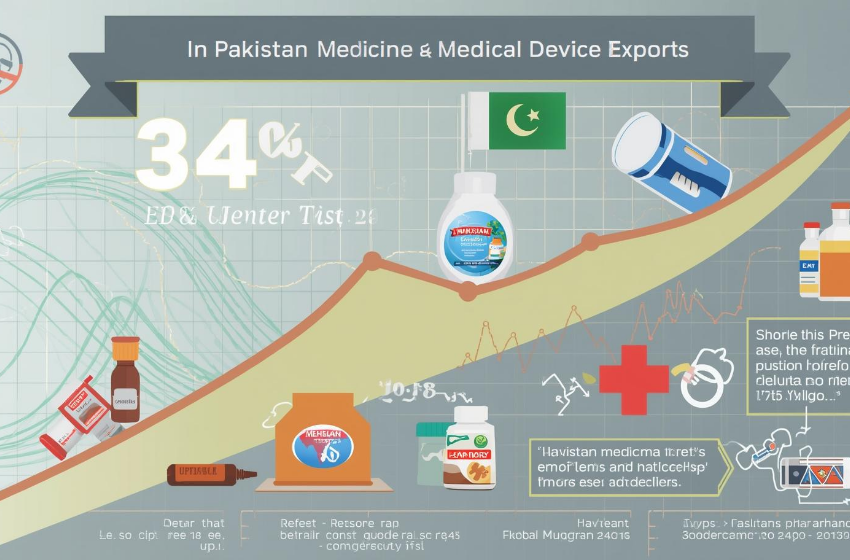
Pakistan Medicine and Medical Device Exports Rise by 34%
Pakistan medicine and medical device exports recorded a strong increase of 34 percent, according to official data shared by the Drug Regulatory Authority of Pakistan (DRAP).
DRAP stated that reforms in drug registration, digital systems, and transparent procedures directly supported this growth. Authorities improved efficiency across regulatory operations to support exporters.
The authority reduced certification timelines to boost exports. Drug export registration now takes only 10 days instead of 60. Medical device registration also completes within 20 days.
Officials confirmed that several export certificates now issue within five days instead of 30. Nearly 70 percent of regulatory work has shifted to digital platforms. DRAP aims to achieve full digitalization by March.
DRAP added that online medical device registration and the e-office system reduced errors and delays. These changes ensured faster access to advanced and life-saving technologies for patients.
Read Also: Influenza Test Kits Shortage Troubles Patients in Lahore
The authority also reduced approval time for new therapies and cancer treatments to three months. Officials are developing a national quality control laboratory network to ensure high drug standards.
Provincial drug testing laboratories are also upgrading to meet international benchmarks. DRAP believes these reforms will improve exports and ensure the supply of safe, effective, and high-quality medicines.
Pakistan medicine and medical device exports are expected to grow further as reforms continue and regulatory efficiency improves nationwide.